


FNH has a scar because it is a hyperplastic process that involves abnormal nodular architecture,
malformed vessels, and cholangiolar proliferation. The scar is composed of fibrous tissue that radiates
from the center of the lesion and separates the nodules. The scar is usually visible on imaging as a
hypodense or hypointense structure that enhances on delayed phases. The scar is a characteristic
feature of FNH, but it is not present in all cases.
FNH is not the only liver lesion that can have a scar. Other liver lesions with a scar include fibrolamellar
carcinoma (FLC), cholangiocarcinoma, hemangioma, and hepatocellular carcinoma (HCC)1. To tell FNH
apart from these lesions, it is important to look at the enhancement pattern of the lesion and the scar
on multiphasic CT and MR imaging1. Some clues that can help differentiate FNH from other liver lesions
with a scar are:
FNH usually shows intense homogeneous enhancement in the arterial phase and isointensity in the
delayed phase, while other lesions may show heterogeneous or rim enhancement1.
FNH shows intense homogeneous enhancement in the arterial phase and isointensity in the delayed
phase because it is composed of normal hepatocytes that have a similar blood supply and function as
the surrounding liver parenchyma1. FNH enhances via the hepatic artery in the arterial phase and then
becomes isointense to the liver in the delayed phase because it has a dual blood supply from both the
hepatic artery and the portal vein2. Other lesions may show heterogeneous or rim enhancement
because they have a different blood supply, composition, or function than the normal liver
parenchyma2. For example, hemangiomas are composed of vascular channels that fill slowly with
contrast, resulting in peripheral nodular enhancement and progressive centripetal fill-in2. HCCs are
composed of malignant hepatocytes that have a higher metabolic activity and blood flow than the
normal liver parenchyma, resulting in hypervascular enhancement in the arterial phase and washout in
the delayed phase2.
FNH usually has a central scar that is hypodense or hypointense on all phases, while other lesions may
have an eccentric scar that can enhance on delayed phases
FNH has a central scar that is hypodense or hypointense on all phases because the scar is composed of
fibrous tissue, cholangiolar proliferation, and malformed vessels that have a lower blood flow and
contrast uptake than the surrounding liver parenchyma12. Other lesions may have an eccentric scar that
can enhance on delayed phases because the scar may contain inflammatory cells, granulation tissue, or
residual tumor cells that have a higher blood flow and contrast uptake than the fibrous tissue34. For
example, fibrolamellar carcinoma may have an eccentric scar that enhances on delayed phases because
it contains tumor cells and inflammatory cells
FNH usually has a large central artery with spoke wheel like centrifugal flow, while other lesions may not
have such a feature.
FNH usually has a large central artery with spoke wheel like centrifugal flow because it is a hyperplastic
lesion that develops around a malformed vessel, possibly an arteriovenous malformation¹². The large
central artery supplies blood to the nodules of FNH and radiates from the center of the lesion to the
periphery, resembling a spoke wheel³⁴. Other lesions may not have such a feature because they have a
different vascular architecture or origin than FNH¹². For example, hemangiomas are composed of
vascular channels that fill slowly with contrast, resulting in peripheral nodular enhancement and
progressive centripetal fill-in².
FNH usually does not have calcifications, cystic components, fat or hemorrhage, while other lesions may
have these features
FNH usually does not have calcifications, cystic components, fat or hemorrhage because it is composed
of nearly normal hepatocytes that have a similar composition and function as the surrounding liver
parenchyma1. FNH is a regenerative lesion that does not undergo necrosis, degeneration, or malignant
transformation1. Other lesions may have these features because they have a different composition,
origin, or behavior than FNH1. For example, hepatic adenomas may have fat or hemorrhage because
they are composed of abnormal hepatocytes that have a higher lipid content and a higher risk of rupture
and bleeding1. Fibrolamellar carcinomas may have calcifications because they are composed of
malignant hepatocytes that have intracellular lamellar inclusions that can calcify2. Fat necrosis may have
cystic components because it is a benign inflammatory process that results from aseptic fat
saponification and can form lipid cysts
FNH usually does not cause portal vein invasion, bile duct dilatation, or lymphadenopathy, while other
lesions may cause these complications
FNH usually does not cause portal vein invasion, bile duct dilatation, or lymphadenopathy because it is a
benign lesion that does not invade or compress the surrounding structures1. FNH does not have a portal
venous supply and does not affect the biliary drainage system1. Other lesions may cause these
complications because they are malignant or inflammatory lesions that can invade or compress the
portal vein, bile ducts, or lymph nodes1. For example, cholangiocarcinoma may cause portal vein
invasion, bile duct dilatation, and lymphadenopathy because it is a malignant tumor that arises from the
biliary epithelium and can grow along the bile ducts or infiltrate the liver parenchyma
Dysembryoplastic neuroepithelial tumors (DNET) are benign (WHO Grade 1) slow growing glioneuronal tumors arising from either cortical or deep grey matter. The vast majority are centered in cortical grey matter, arise from secondary germinal layers, and are frequently associated with cortical dysplasia (in up to 80% of cases). They characteristically cause intractable focal seizures.
Patients with DNETs typically present with longstanding treatment-resistant focal seizures (in 90% of cases the first seizure occurred before the age of 20).
The most common treatment for DNET is surgical removal of the tumor. Because it is a benign tumor, and prognosis is good even if not the entire tumor is removed, radiation and chemotherapy are not typically used.
T1-weighted images with gadolinium: DNETs typically appear as well-defined, non-enhancing lesions. This means that they do not take up the contrast agent, resulting in little to no enhancement compared to the surrounding brain tissue.
T2-weighted and FLAIR (fluid-attenuated inversion recovery) images: DNETs often exhibit a characteristic appearance on these sequences. They appear as well-circumscribed, nodular masses with a mixed or heterogeneous signal intensity. The tumor may have areas of both high and low signal intensity due to the presence of different tissue components within the tumor.
T2 FLAIR
T2W
On MRI, cardioembolic strokes are often identified by their involvement in a cortical territory. Bilateral infarcts and multiple territory infarcts are more common in patients with a cardioembolic source of stroke than patients without a cardioembolic source
In radiology, cardioembolic strokes are most often identified by their involvement in a cortical territory. About half of cardioembolic strokes involve multiple cerebral arterial territories
The MRI findings in a cardioembolic infarction can vary depending on the location and size of the infarcted area in the brain. MRI (magnetic resonance imaging) is a sensitive imaging technique that can provide detailed information about the structure and function of the brain. Here are some common MRI findings associated with cardioembolic infarctions:
It’s important to note that the MRI findings can also help differentiate a cardioembolic infarction from other types of strokes or brain conditions. The specific MRI findings and their interpretation should be assessed by a radiologist or a healthcare professional experienced in reading and interpreting neuroimaging studies. They will consider the clinical history, physical examination, and other diagnostic tests to arrive at a definitive diagnosis and guide appropriate treatment.

This is an acute infarction
Ðường rách gan HPT IVb sâu 28mm tạo khối máu tụ bờ dưới gan kt# 60x85mm kèm rách đáy túi mật gây xuất huyết trong lòng túi mật. Không giãn đường mật trong và ngoài gan.
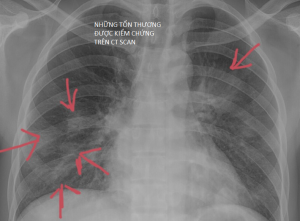
Quan sát kĩ 2 phế trường ghi nhận các tổn thương dạng nốt và đám mờ có hình airbronchogram bên trong tổn thương thuỳ trên phổi (t).
Trên CT Scan các tổn thương viêm thường vẫn tôn trọng rãnh liên thùy. Ngược lại các tổn thương u thường vượt qua ranh giới này
Click vào xem kết quả ct scan ngực
KQ CT Scan ngực có cản quang
Định nghĩa
Sự phát triển bất thường tĩnh mạch (DVA), còn được gọi là u mạch tĩnh mạch não là một dị dạng bẩm sinh của các tĩnh mạch bình thường của não. Trước khi các kỹ thuật cắt lớp ra đời thì nó được cho là 1 bệnh hiếm gặp, tuy nhiên hiện đã được công nhận là dị dạng mạch máu não phổ biến nhất, chiếm ~ 55% (khoảng 50-63%) của tất cả các tổn thương.
DVA được đặc trưng bởi dấu hiệu đầu sứa (Xem hình minh hoạ) hợp lưu vào 1 tĩnh mạch lớn riêng biệt dẫn lưu về tĩnh mạch màng cứng hoặc vào tĩnh mạch nội tuỷ sâu. Dấu hiệu này giống với hình cành cây cọ.
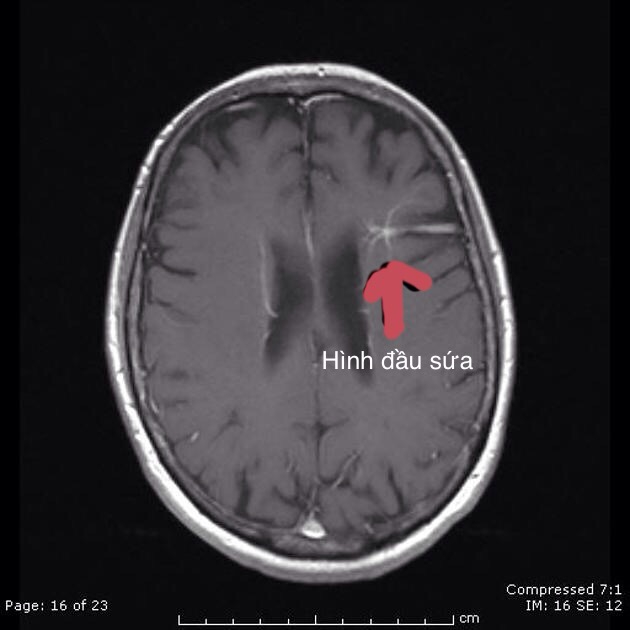
Hình ảnh đầu sứa trong DVA
Continue reading